Domain Theory Of Paramagnetism
Next week will look at this phenomena in more detail using the domain theory of ferromagnetism.
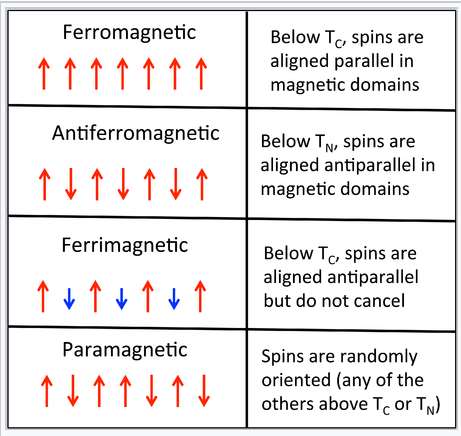
Domain theory of paramagnetism. The domain theory of magnetism. Saw magnetization was a function of previous history. However for materials that show some other form of magnetism such as ferromagnetism or paramagnetism the diamagnetic contribution becomes negligible. Magnetic domain theory was developed by french physicist pierre ernest weiss who in 1906 suggested existence of magnetic domains in ferromagnets.
The domain theory of ferromagnetism in a paramagnet the increasing magnetisation m is due to the increasing alignment of the magnetic dipoles in the µ b kt magnetic versus thermal competition for a ferromagnet extremely large values of m can be created by the application of very small applied fields h. Development of domain theory. If even one orbital has a net spin the entire atom will have a net spin. Some atoms or ions in the material have a net magentic moment due to unpaired electrons in partially filled orbitals.
Diamagnetism to a greater or lesser degree is a property of all materials and will always make a weak contribution to the material s response to a magnetic field. Electrons that are alone in an orbital are called paramagnetic electrons. Ferromagnetism is a special case of paramagnetism. In contrast with this behavior diamagnetic materials are repelled by magnetic fields and form induced magnetic fields in the direction opposite to that of the applied magnetic field.
Remember that if an electron is alone in an orbital the orbital has a net spin because the spin of the lone electron does not get canceled out. Derived a quantum theory of paramagnetism. Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field and form internal induced magnetic fields in the direction of the applied magnetic field. The direction of alignment varies from domain to domain in a more or less random manner.
In ferromagnetic substances to the magnetic dipole moment of atoms the contribution of the spin magnetic moment is very large. Paramagnetic materials such as liquid oxygen and aluminium show a weak magnetic attraction when placed near a magnet. He suggested that large number of atomic magnetic moments typically 10 12 10 18 citation needed were aligned parallel.













